When setting up a fractional distillation, it is common practice to just "grab whatever column is on the rack." Whether it’s a Vigreux or a random packed column, the logic is often: "If it fits the flask, it works."
But if you are facing these common lab nightmares:
- Poor Separation: Two components with a 10°C boiling point difference just won't separate.
- Instability: The column floods, dries out, and the temperature reading fluctuates wildly.
- Time Loss: The column is too long, the reflux is aggressive, and a simple run takes all day with low recovery.
The problem isn’t usually your chemistry—it’s your hardware choice.
In this guide, we skip the complex chemical engineering equations. (For a deep dive into the internal mechanics, we recommend reading Chemnorth's Inside a Fractional Distillation Column: A Visual Guide to 4 Lab Types). Instead, we answer one practical question: How do you select the "just right" fractionating column for your specific experiment?
We will break down the four most common laboratory columns:
- Vigreux Column (The Rugged Standard)
- Snyder Column (The Visual Workhorse)
- Packed Column (The Efficiency Expert – includes Hempel)
- Spinning Band Column (The Ultimate Weapon)
Phase 1: The Pre-Check (Ask Before You Grab)
Before looking at a catalog, ask yourself these four questions. Your answers define your equipment.
1. What is the Boiling Point Difference (Δ\DeltaΔBP)?
- > 30 °C: Easy separation.
- 10–30 °C: Moderate difficulty.
- < 10 °C: Difficult / High precision required.
2. What is the Sample Volume?
- Micro-scale: Every drop counts (Low Holdup required).
- 50–200 mL: Standard teaching/lab scale.
- Macro-scale: > 500 mL (High throughput required).
3. What is the Goal?
- Rough Separation: Just removing solvent or separating distinct layers.
- High Purity: Need analytical grade purity.
- High Recovery: Cannot afford to lose material in the column packing.
4. Constraints?
- Vacuum requirements? Thermal sensitivity? Budget?
Phase 2: The 4 Types of Columns Explained
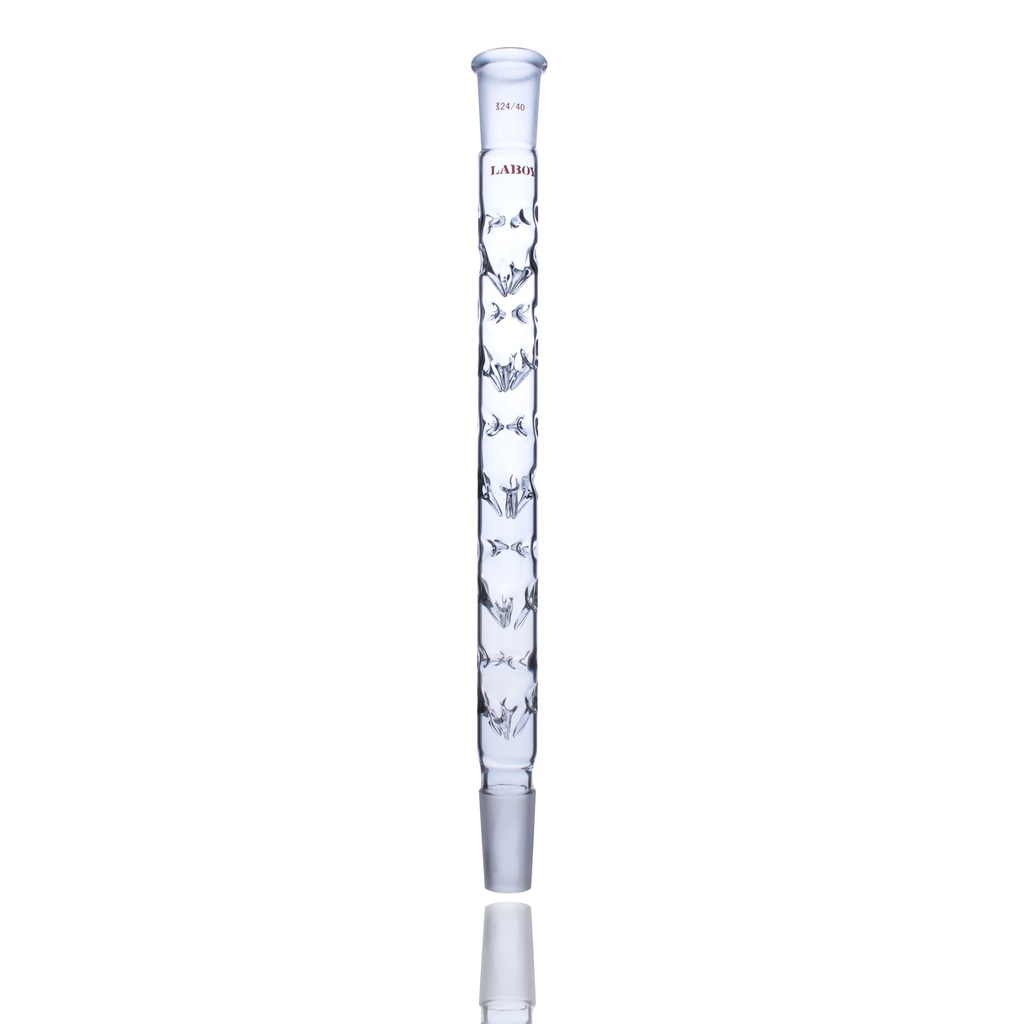
1. Vigreux Column
The "Good Enough" Standard
Best for: Teaching, Solvent Recovery, Wide ΔBP
Structure
A glass tube featuring rows of downward-pointing glass indentations (spikes). These spikes increase the surface area without restricting air flow.
When to use it
- General purpose distillation.
- Undergraduate teaching labs.
- Purifying solvents with wide boiling point differences (>20°C).
Pros
- Robust: No loose parts to break.
- Low Hold-up: Very little liquid gets stuck inside.
- Cheap: Most affordable option.
Cons
Low efficiency. It has a low number of theoretical plates, making it unsuitable for separating closely boiling compounds.
1. Vigreux Column
The "Good Enough" Standard
Best for: Teaching, Solvent Recovery, Wide ΔBP
Structure
A glass tube featuring rows of downward-pointing glass indentations (spikes). These spikes increase the surface area without restricting air flow.
When to use it
- General purpose distillation.
- Undergraduate teaching labs.
- Purifying solvents with wide boiling point differences (>20°C).
Pros
- Robust: No loose parts to break.
- Low Hold-up: Very little liquid gets stuck inside.
- Cheap: Most affordable option.
Cons
Low efficiency. It has a low number of theoretical plates, making it unsuitable for separating closely boiling compounds.
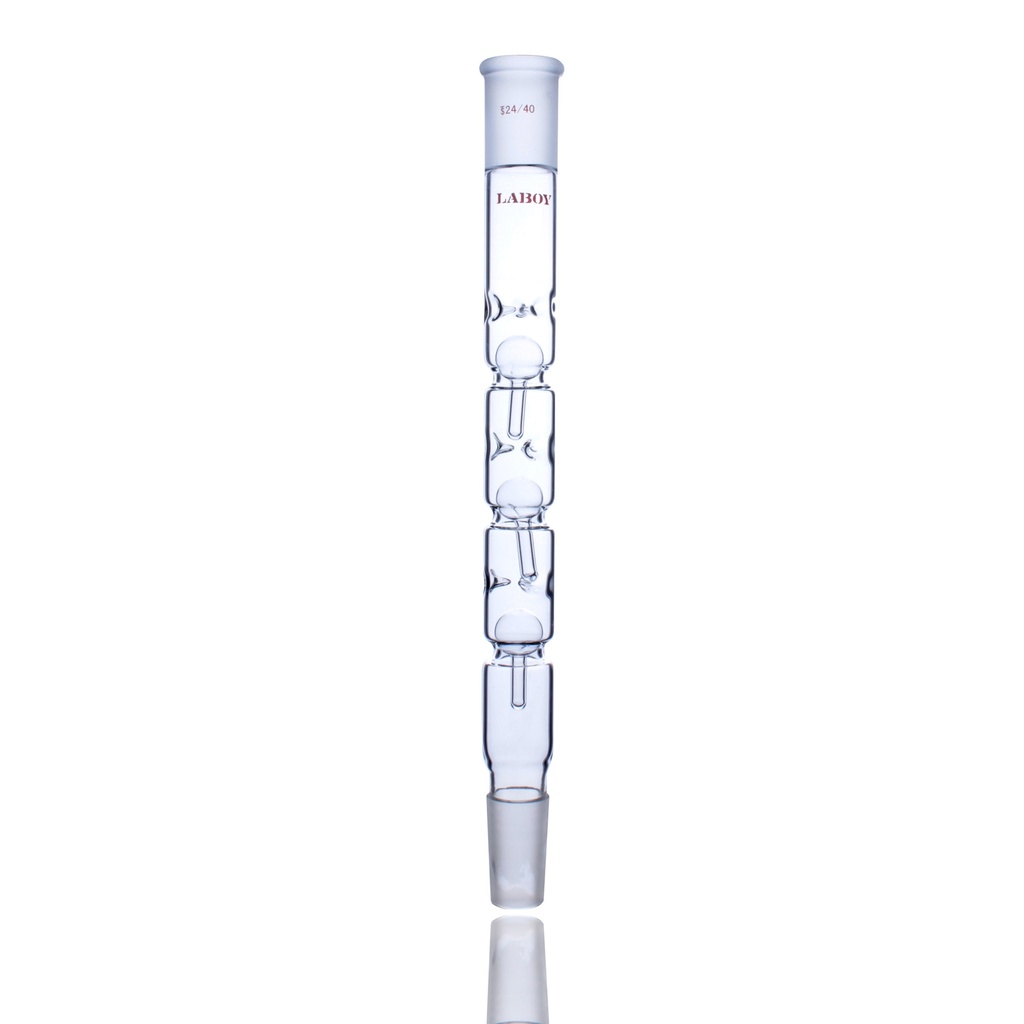
2. Snyder Column
The Visual & Anti-Bump Choice
Best for: Solvent Concentration (Kuderna-Danish), Bumping Solvents
Structure
A wide glass tube divided into vertical chambers by glass constrictions. Inside each chamber sits a loose, hollow glass ball. As vapor rises, it lifts the balls, causing them to float and rattle.
When to use it
- Solvent Concentration: Essential for Kuderna-Danish (K-D) evaporators to rapidly remove solvents.
- Problematic Liquids: Distilling mixtures that tend to "bump" (boil violently) or foam.
Pros
- Anti-Bumping: The floating balls act like mechanical boiling chips, breaking up large bubbles.
- High Throughput: Can handle a very large volume of vapor quickly without choking.
- Visual Feedback: You can see the balls moving, making it easy to judge the distillation rate.
Cons
- Fragile: The loose glass balls can break if the column is shaken or dropped.
- Hard to Clean: If solids crystallize inside the chambers, they are very difficult to remove.
- Flooding: If heated too fast, liquid can get trapped above the balls.
2. Snyder Column
The Visual & Anti-Bump Choice
Best for: Solvent Concentration (Kuderna-Danish), Bumping Solvents
Structure
A wide glass tube divided into vertical chambers by glass constrictions. Inside each chamber sits a loose, hollow glass ball. As vapor rises, it lifts the balls, causing them to float and rattle.
When to use it
- Solvent Concentration: Essential for Kuderna-Danish (K-D) evaporators to rapidly remove solvents.
- Problematic Liquids: Distilling mixtures that tend to "bump" (boil violently) or foam.
Pros
- Anti-Bumping: The floating balls act like mechanical boiling chips, breaking up large bubbles.
- High Throughput: Can handle a very large volume of vapor quickly without choking.
- Visual Feedback: You can see the balls moving, making it easy to judge the distillation rate.
Cons
- Fragile: The loose glass balls can break if the column is shaken or dropped.
- Hard to Clean: If solids crystallize inside the chambers, they are very difficult to remove.
- Flooding: If heated too fast, liquid can get trapped above the balls.
3. Packed Column: The Efficiency Powerhouse
Best for: High Purity, Close Boiling Points (10–30 °C)
3. Packed Column
The Efficiency Powerhouse
Best for: High Purity, Close Boiling Points (10–30 °C)
Structure
A plain glass tube filled with small, solid objects known as "packing material" (e.g., glass beads, Raschig rings, or glass helices). The vapor must navigate around these obstacles.
When to use it
- High Purity Needs: When you need better separation than a Vigreux column can provide.
- Close Boiling Points: Ideal for separating liquids with boiling point differences between 10°C and 30°C.
Pros
- High Efficiency: Provides a massive surface area for vapor-liquid exchange, resulting in many theoretical plates.
- Versatile: You can change the packing material (glass, ceramic, steel) to suit different chemicals.
Cons
- High Hold-up: A significant amount of liquid stays coating the packing material and is lost.
- Risk of Flooding: If heated too quickly, liquid gets trapped in the packing, causing a "traffic jam" (flooding).
- Hard to Clean: Cleaning inside the tiny packing beads is very difficult.
3. Packed Column
The Efficiency Powerhouse
Best for: High Purity, Close Boiling Points (10–30 °C)
Structure
A plain glass tube filled with small, solid objects known as "packing material" (e.g., glass beads, Raschig rings, or glass helices). The vapor must navigate around these obstacles.
When to use it
- High Purity Needs: When you need better separation than a Vigreux column can provide.
- Close Boiling Points: Ideal for separating liquids with boiling point differences between 10°C and 30°C.
Pros
- High Efficiency: Provides a massive surface area for vapor-liquid exchange, resulting in many theoretical plates.
- Versatile: You can change the packing material (glass, ceramic, steel) to suit different chemicals.
Cons
- High Hold-up: A significant amount of liquid stays coating the packing material and is lost.
- Risk of Flooding: If heated too quickly, liquid gets trapped in the packing, causing a "traffic jam" (flooding).
- Hard to Clean: Cleaning inside the tiny packing beads is very difficult.
4. Spinning Band Column: The "Money is No Object" Solution
Best for: Research Grade Purity, Δ\DeltaΔBP < 10 °C, Precious Samples
- Structure: A precision-bore tube with a helical metal or Teflon band rotating inside, driven by a motor. The rotation whips the liquid into a thin film, maximizing surface area.
- When to use it:
- Boiling point differences are tiny (< 10 °C).
- Sample is extremely valuable (needs lowest possible hold-up).
- High-vacuum distillation where pressure drop must be minimized.
- Pros:
- Maximum Efficiency: The highest theoretical plate count available for lab scale.
- Low Hold-up: You recover almost all your material.
- Cons: Expensive equipment; requires mechanical setup (motor/controller); moving parts can wear out.
- Verdict: "When nothing else works, or the sample is worth more than the equipment, bring out the Spinning Band."
Comparison Cheat Sheet (Quick Selection Guide)
| Scenario | Recommended Column | Why? |
|---|
| Teaching / Simple Solvent Removal | Vigreux | Rugged, cheap, easiest to clean. |
| Bumping Liquids / Solvent Concentration | Snyder | Handles bumping well, high throughput, visual feedback. |
| Standard Purification (Δ\DeltaΔBP 15–30°C) | Packed (e.g., Hempel) | Good balance of efficiency and cost. |
| High Purity / Precious Sample (Δ\DeltaΔBP <10°C) | Spinning Band | Highest efficiency, lowest sample loss. |
| Beginner Chemist | Vigreux | Hard to break, hard to mess up. |
Conclusion: Stop Guessing, Start Designing
At ChemNorth, we believe that the glassware should fit the chemistry, not the other way around.
Don't just use the longest column you can find.
- If you are just stripping ether, a Snyder or Vigreux is faster and better.
- If you are separating close isomers, a Packed column is mandatory.
- If you are refining a few grams of a high-value intermediate, invest in Spinning Band.
Choose wisely, and may your fractions always be pure.